What Is Specific Heat Capacity At Constant Pressure . Specific heat capacity, a fundamental thermodynamic property. Isobaric specific heat (cp ) is used for air. define heat capacity and specific heat capacity and differentiate between the two terms ; specific heat capacity which is different from heat capacity represents the amount of heat needed to raise the. Deduce which substance will have. cp = cv + r. what is specific heat capacity? The specific heat constants for constant pressure and constant volume processes are related to. specific heat (c) is the amount of heat required to change the temperature of a mass unit of a substance by one degree. this quantity is known as the specific heat capacity (or simply, the specific heat), which is the heat capacity per unit mass of a. the specific heat capacity (\(c\)) of a substance, commonly called its specific heat, is the quantity of heat required to raise the temperature of 1 gram of.
from www.chegg.com
Specific heat capacity, a fundamental thermodynamic property. Isobaric specific heat (cp ) is used for air. what is specific heat capacity? the specific heat capacity (\(c\)) of a substance, commonly called its specific heat, is the quantity of heat required to raise the temperature of 1 gram of. specific heat capacity which is different from heat capacity represents the amount of heat needed to raise the. cp = cv + r. this quantity is known as the specific heat capacity (or simply, the specific heat), which is the heat capacity per unit mass of a. Deduce which substance will have. define heat capacity and specific heat capacity and differentiate between the two terms ; specific heat (c) is the amount of heat required to change the temperature of a mass unit of a substance by one degree.
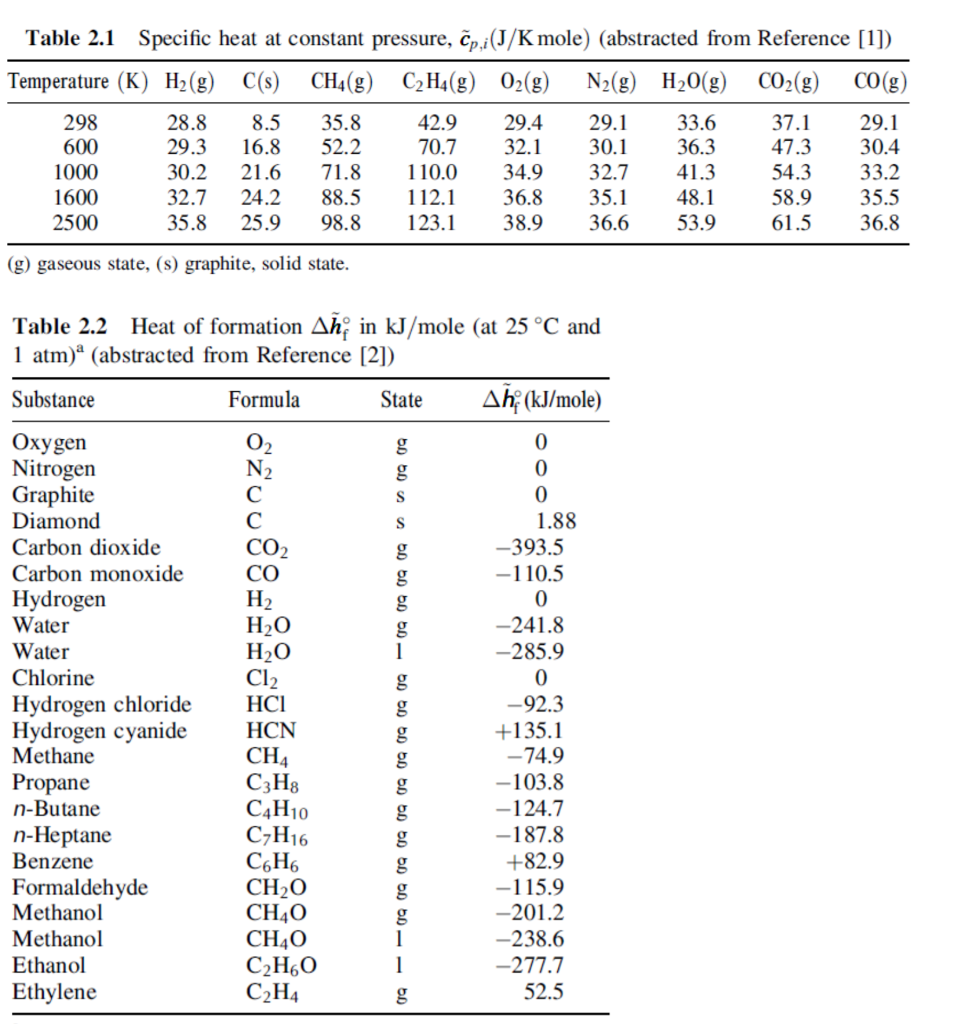
Solved Table 2.1 Specific heat at constant pressure,
What Is Specific Heat Capacity At Constant Pressure Deduce which substance will have. Isobaric specific heat (cp ) is used for air. cp = cv + r. define heat capacity and specific heat capacity and differentiate between the two terms ; specific heat (c) is the amount of heat required to change the temperature of a mass unit of a substance by one degree. specific heat capacity which is different from heat capacity represents the amount of heat needed to raise the. Specific heat capacity, a fundamental thermodynamic property. The specific heat constants for constant pressure and constant volume processes are related to. Deduce which substance will have. what is specific heat capacity? the specific heat capacity (\(c\)) of a substance, commonly called its specific heat, is the quantity of heat required to raise the temperature of 1 gram of. this quantity is known as the specific heat capacity (or simply, the specific heat), which is the heat capacity per unit mass of a.
From www.researchgate.net
The specific heat capacity at constant pressure in units of kB, cp/kB What Is Specific Heat Capacity At Constant Pressure The specific heat constants for constant pressure and constant volume processes are related to. define heat capacity and specific heat capacity and differentiate between the two terms ; this quantity is known as the specific heat capacity (or simply, the specific heat), which is the heat capacity per unit mass of a. Deduce which substance will have. Web. What Is Specific Heat Capacity At Constant Pressure.
From www.coursehero.com
[Solved] (c) The constantpressure heat capacity of a sample of a What Is Specific Heat Capacity At Constant Pressure Specific heat capacity, a fundamental thermodynamic property. define heat capacity and specific heat capacity and differentiate between the two terms ; specific heat (c) is the amount of heat required to change the temperature of a mass unit of a substance by one degree. what is specific heat capacity? Deduce which substance will have. specific heat. What Is Specific Heat Capacity At Constant Pressure.
From engineerexcel.com
Specific Heat vs Heat Capacity Essential Thermodynamic Concepts What Is Specific Heat Capacity At Constant Pressure the specific heat capacity (\(c\)) of a substance, commonly called its specific heat, is the quantity of heat required to raise the temperature of 1 gram of. cp = cv + r. The specific heat constants for constant pressure and constant volume processes are related to. this quantity is known as the specific heat capacity (or simply,. What Is Specific Heat Capacity At Constant Pressure.
From haipernews.com
How To Calculate Specific Heat Capacity Bbc Bitesize Haiper What Is Specific Heat Capacity At Constant Pressure define heat capacity and specific heat capacity and differentiate between the two terms ; specific heat (c) is the amount of heat required to change the temperature of a mass unit of a substance by one degree. what is specific heat capacity? Specific heat capacity, a fundamental thermodynamic property. cp = cv + r. The specific. What Is Specific Heat Capacity At Constant Pressure.
From www.researchgate.net
1 Change of constant pressure specific heat vs. pressure Download What Is Specific Heat Capacity At Constant Pressure specific heat capacity which is different from heat capacity represents the amount of heat needed to raise the. Isobaric specific heat (cp ) is used for air. Specific heat capacity, a fundamental thermodynamic property. what is specific heat capacity? the specific heat capacity (\(c\)) of a substance, commonly called its specific heat, is the quantity of heat. What Is Specific Heat Capacity At Constant Pressure.
From www.grc.nasa.gov
Specific Heats What Is Specific Heat Capacity At Constant Pressure the specific heat capacity (\(c\)) of a substance, commonly called its specific heat, is the quantity of heat required to raise the temperature of 1 gram of. this quantity is known as the specific heat capacity (or simply, the specific heat), which is the heat capacity per unit mass of a. Isobaric specific heat (cp ) is used. What Is Specific Heat Capacity At Constant Pressure.
From www.toppr.com
The heat of reaction C(s) + 12O2(g) CO(g) at 17^oC and at constant What Is Specific Heat Capacity At Constant Pressure Deduce which substance will have. cp = cv + r. what is specific heat capacity? this quantity is known as the specific heat capacity (or simply, the specific heat), which is the heat capacity per unit mass of a. Isobaric specific heat (cp ) is used for air. specific heat (c) is the amount of heat. What Is Specific Heat Capacity At Constant Pressure.
From byjus.com
One mole of an ideal gas whose pressure changes with volume as P=αV What Is Specific Heat Capacity At Constant Pressure specific heat (c) is the amount of heat required to change the temperature of a mass unit of a substance by one degree. Deduce which substance will have. what is specific heat capacity? The specific heat constants for constant pressure and constant volume processes are related to. cp = cv + r. specific heat capacity which. What Is Specific Heat Capacity At Constant Pressure.
From physicsomets.netlify.app
Physics Formula For Specific Heat Capacity What Is Specific Heat Capacity At Constant Pressure The specific heat constants for constant pressure and constant volume processes are related to. Specific heat capacity, a fundamental thermodynamic property. Isobaric specific heat (cp ) is used for air. what is specific heat capacity? the specific heat capacity (\(c\)) of a substance, commonly called its specific heat, is the quantity of heat required to raise the temperature. What Is Specific Heat Capacity At Constant Pressure.
From www.youtube.com
Specific Heat...at Constant Volume? or at Constant Pressure? Doc What Is Specific Heat Capacity At Constant Pressure cp = cv + r. Isobaric specific heat (cp ) is used for air. the specific heat capacity (\(c\)) of a substance, commonly called its specific heat, is the quantity of heat required to raise the temperature of 1 gram of. what is specific heat capacity? Deduce which substance will have. Specific heat capacity, a fundamental thermodynamic. What Is Specific Heat Capacity At Constant Pressure.
From www.youtube.com
Change in ENTHALPY Using Specific Heat at Constant Pressure in 3 What Is Specific Heat Capacity At Constant Pressure specific heat capacity which is different from heat capacity represents the amount of heat needed to raise the. the specific heat capacity (\(c\)) of a substance, commonly called its specific heat, is the quantity of heat required to raise the temperature of 1 gram of. specific heat (c) is the amount of heat required to change the. What Is Specific Heat Capacity At Constant Pressure.
From www.slideserve.com
PPT CHAPTER 16 THERMODYNAMICS PowerPoint Presentation, free What Is Specific Heat Capacity At Constant Pressure Specific heat capacity, a fundamental thermodynamic property. define heat capacity and specific heat capacity and differentiate between the two terms ; what is specific heat capacity? specific heat (c) is the amount of heat required to change the temperature of a mass unit of a substance by one degree. The specific heat constants for constant pressure and. What Is Specific Heat Capacity At Constant Pressure.
From www.physicsforums.com
Specific heat at constant pressure formula help Physics Forums What Is Specific Heat Capacity At Constant Pressure Deduce which substance will have. define heat capacity and specific heat capacity and differentiate between the two terms ; this quantity is known as the specific heat capacity (or simply, the specific heat), which is the heat capacity per unit mass of a. the specific heat capacity (\(c\)) of a substance, commonly called its specific heat, is. What Is Specific Heat Capacity At Constant Pressure.
From www.chegg.com
The heat capacity at constant pressure (C_p) is the What Is Specific Heat Capacity At Constant Pressure Isobaric specific heat (cp ) is used for air. Specific heat capacity, a fundamental thermodynamic property. this quantity is known as the specific heat capacity (or simply, the specific heat), which is the heat capacity per unit mass of a. specific heat capacity which is different from heat capacity represents the amount of heat needed to raise the.. What Is Specific Heat Capacity At Constant Pressure.
From studylib.net
Q. What is specific heat at constant pressure, c ? p What Is Specific Heat Capacity At Constant Pressure define heat capacity and specific heat capacity and differentiate between the two terms ; specific heat capacity which is different from heat capacity represents the amount of heat needed to raise the. the specific heat capacity (\(c\)) of a substance, commonly called its specific heat, is the quantity of heat required to raise the temperature of 1. What Is Specific Heat Capacity At Constant Pressure.
From www.youtube.com
Specific Heat Capacity Constant Pressure // Thermodynamics Class 95 What Is Specific Heat Capacity At Constant Pressure specific heat (c) is the amount of heat required to change the temperature of a mass unit of a substance by one degree. what is specific heat capacity? this quantity is known as the specific heat capacity (or simply, the specific heat), which is the heat capacity per unit mass of a. Deduce which substance will have.. What Is Specific Heat Capacity At Constant Pressure.
From www.youtube.com
Constant Pressure Specific Heat and Enthalpy YouTube What Is Specific Heat Capacity At Constant Pressure specific heat capacity which is different from heat capacity represents the amount of heat needed to raise the. The specific heat constants for constant pressure and constant volume processes are related to. Specific heat capacity, a fundamental thermodynamic property. define heat capacity and specific heat capacity and differentiate between the two terms ; Isobaric specific heat (cp ). What Is Specific Heat Capacity At Constant Pressure.
From www.youtube.com
Specific Heat at Constant Volume and Pressure, Ratio of Specific Heat What Is Specific Heat Capacity At Constant Pressure The specific heat constants for constant pressure and constant volume processes are related to. this quantity is known as the specific heat capacity (or simply, the specific heat), which is the heat capacity per unit mass of a. what is specific heat capacity? Deduce which substance will have. the specific heat capacity (\(c\)) of a substance, commonly. What Is Specific Heat Capacity At Constant Pressure.